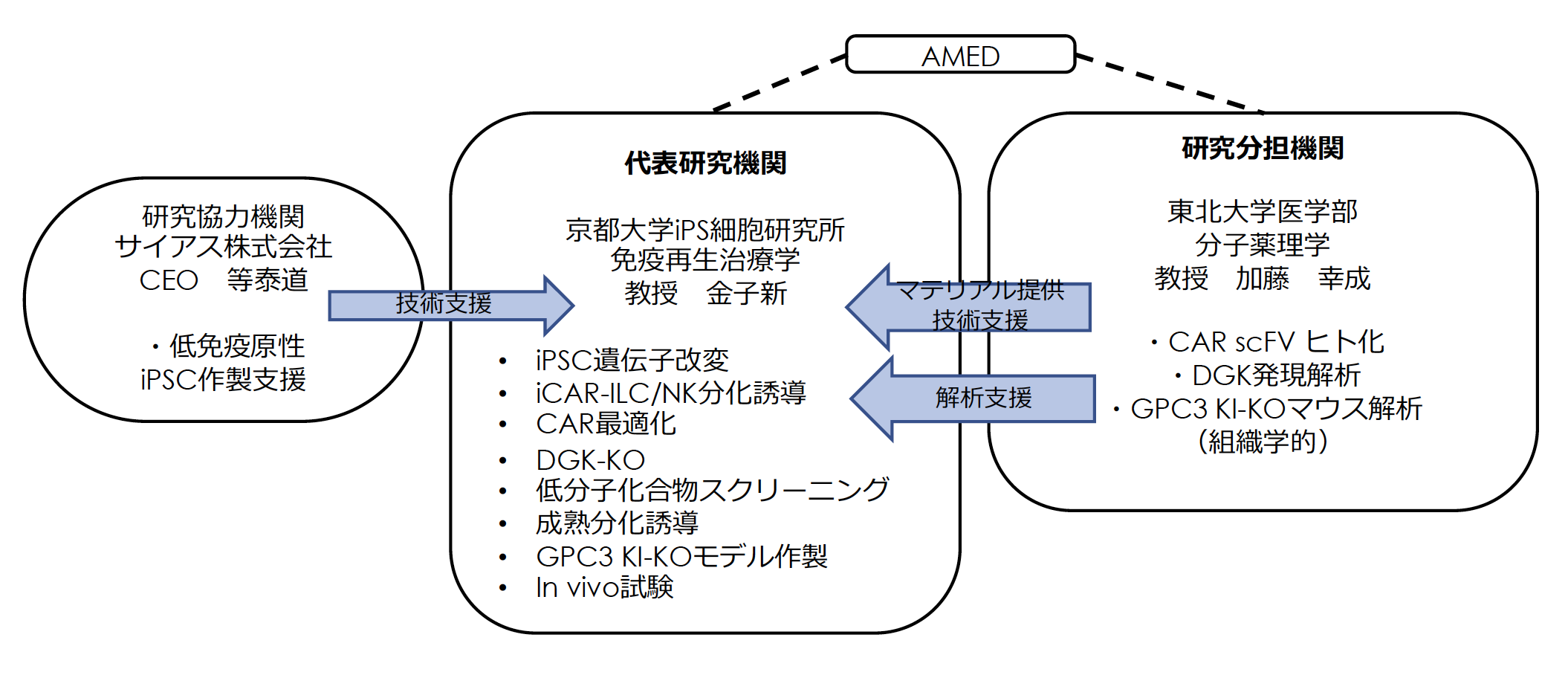
再生・細胞医療・遺伝子治療実現加速化プログラム
再生医療(AMED) 分担研究者:東北大学 加藤幸成(代表:京都大学 金子 新 )
<文献>
2025
Satofuka H, Suzuki H, Tanaka T, Li G, Kaneko MK, Kato Y. Biochem Biophys Rep , 42, 1019982024, 2025 (PDF ; preprint )
VIDEO
Li G, Suzuki H, Tanaka T, Satofuka H, Kaneko MK, Kato Y. Biochem Biophys Rep , 42, 101965, 2025 (PDF ; preprint )
VIDEO
Tanaka T, Kaneko Y, Yamamoto H, Li G, Fujisawa S, Satofuka H, Shinoda K, Nakamura T, Kaneko MK, Suzuki H, Kato Y. Biochem Biophys Rep , 41, 101960, https://doi.org/10.1016/j.bbrep.2025.101960, 2024 (PDF ; preprint )
Satofuka H, Suzuki H, Tanaka T, Ubukata R, Hirose M, Yamamoto H, Kaneko Y, Fujisawa S, Li G, Kaneko MK, Kato Y. Biochem Biophys Rep , 41, 101948, https://doi.org/10.1016/j.bbrep.2025.101948, 2025 (PDF ; preprint )
VIDEO
Ishikawa K, Suzuki H,Tanaka T,Kaneko MK, Kato Y. MI , 2(1), 101–113; https://doi.org/10.36922/mi.5664, 2025 (PDF ; preprint )
VIDEO
Kaneko MK, Suzuki H, Ohishi T, Nakamura T, Yanaka M, Tanaka T, Kato Y. Int. J. Mol. Sci. , 2025(PDF ; preprint )
VIDEO
Mishima Y, Okada S, Ishikawa A, Wang B, Waseda M, Kaneko MK, Kato Y, Kaneko S. PDF )
2024
Tanaka T, Suzuki H, Ohishi T, Kaneko MK, Kato Y. PDF ; preprint )VIDEO
Hirose M, Suzuki H, Ubukata R, Tanaka T, Kaneko MK, Kato Y. Biochem Biophys Rep , 40, 101824, https://doi.org/10.1016/j.bbrep.2024.101824, 2024 (PDF ; preprint )
VIDEO
Ishikawa K, Suzuki H, Ohishi T, Nakamura T, Yanaka M, Li G, Tanaka T, Kawada M, Kaneko MK, Ohkoshi A, Katori Y, Kato Y.
Oncology reports , 52(5), 147, https://doi.org/10.3892/or.2024.8806, (PDF ; preprint )VIDEO
Ishikawa K, Suzuki H, Ohishi T, Li G, Tanaka T, Kawada M, Ohkoshi A, Kaneko MK, Katori Y, Kato Y. Int. J. Mol. Sci. , 25(17), 9190; https://doi.org/10.3390/ijms25179190, 2024 (PDF ; preprint )
VIDEO
Ubukata R, Suzuki H, Tanaka T, Li G, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(4), 112-118, https://doi.org/10.1089/mab.2024.0009, 2024 (PDF )
Suzuki H, Tanaka T, Li G, Ouchida T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(4), 96-100, https://doi.org/10.1089/mab.2024.0004, 2024 (PDF ; preprint )
Kobayashi H, Suzuki H, Tanaka T, Kaneko MK, Kato Y. Monoclon. Antib. Immunodiagn. Immunother. , 43(4), 101-107, https://doi.org/10.1089/mab.2024.0002, 2024 (PDF ; preprint )
Suzuki H, Ohishi T, Tanaka T, Kaneko MK, Kato Y.
Int. J. Mol. Sci. , 25(15), 8386; https://doi.org/10.3390/ijms25158386, 2024 (PDF ; preprint )
VIDEO
Arimori T, Mihara E, Suzuki H, Ohishi T, Tanaka T, Kaneko MK, Takagi J, Kato Y.
Structure , 32(5),536-549, https://doi.org/10.1016/j.str.2024.02.007, 2024 (PDF )
VIDEO
Li G, Tanaka T, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 59-66, DOI: 10.20944/preprints202311.0501.v2, 2023 (PDF ;preprint )
Ouchida T, Li G, Suzuki H, Yanaka M, Nakamura T, Handa S, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 53-58, doi:10.1089/mab.2024.0003, 2024 (PDF )
Kaneko MK, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 35-43,
DOI: 10.1089/mab.2023.0033, 2024 (PDF ;online ;preprint )
Ouchida T, Isoda Y, Nakamura T, Yanaka M, Tanaka T, Handa S, Kaneko MK, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 67-74, https://doi.org/10.1089/mab.2023.0032, 2024 (PDF ;preprint )
Okada Y, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,43(2), 44-52, https://doi.org/10.1089/mab.2023.0029, 2024 (PDF ;preprint )
Inoue T, Yamamoto Y, Sato K, Nakamura Y, Shimizu Y, Ogawa M, Onodera T, Takahashi Y, Wakita T, Kaneko MK, Fukasawa M, Kato Y, Noguchi K.
iScience ,27(4),109363, DOI:https://doi.org/10.1016/j.isci.2024.109363, 2024 (PDF )
Ouchida T, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(1), 17–23, https://doi.org/10.1089/mab.2023.0014, 2024 (PDF ; preprint )
Okada Y, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(1), 24–31, https://doi.org/10.1089/mab.2023.0016, 2024 (PDF ; preprint )
Ouchida T, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 43(1), 10–16, DOI: 10.1089/mab.2023.0023, 2024 (PDF ;preprint )
Kaneko MK, Suzuki H, Ohishi T, Nakamura T, Tanaka T, Kato Y.
Int. J. Mol. Sci. , 25(3), 1941; https://doi.org/10.3390/ijms25031941, 2024 (PDF )
Tanaka T, Suzuki H, Ohishi T, Kaneko MK, Kato Y.
Cancer Sci. , 115(1), 298-309, https://doi.org/10.1111/cas.16008, 2024 (PDF ; preprint )
Suzuki H, Ohishi T, Tanaka T Kaneko MK, Kato Y.
Int. J. Mol. Sci. , 25, 161. https://doi.org/10.3390/ijms25010161, 2024 (PDF ; preprint )
2023
Isoda Y, Kaneko MK, Tanaka T, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(6), 189–193, DOI: 10.1089/mab.2023.0026, 2023 (PDF ; preprint )
Ouchida T, Tanaka T, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(6), 209–215, https://doi.org/10.1089/mab.2023.0020, 2023 (PDF ; preprint )
Suzuki H, Tanaka T, Kudo Y, Tawara M, Hirayama A, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(6), 203–208, DOI: 10.1089/mab.2023.0018, 2023 (PDF ; preprint )
Nanamiya R, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,42(5), 153–156, https://doi.org/10.1089/mab.2023.0015, 2023 (PDF ; preprint )
Suzuki H, Ohishi T, Kaneko MK, Kato Y.
Cancers , 15(20), 5080; https://doi.org/10.3390/cancers15205080, 2023 (PDF )
Suzuki H, Ohishi T, Nanamiya R, Kawada M, Kaneko MK, Kato Y.
Curr. Issues Mol. Biol. , 45(10), 7734-7748; https://doi.org/10.3390/cimb45100488, 2023 (PDF )
Ouchida T, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Int. J. Transl. Med. , 3(3), 310-320; 2023 (PDF )
動画(YouTube)
総説(pdf)
AMED project
再生医療実現拠点ネットワークプログラム
再生医療(AMED) 分担研究者:東北大学 加藤幸成(代表:京都大学 金子 新 )
<文献>
2023
Suzuki H, Ohishi T, Nanamiya R, Kawada M, Kaneko MK, Kato Y.
Curr. Issues Mol. Biol. , 45(10), 7734-7748; https://doi.org/10.3390/cimb45100488, 2023 (PDF )
Suzuki H, Goto N, Tanaka T, Ouchida T, Kaneko MK, Kato Y.
antibodies , 12(3), 45; https://doi.org/10.3390/antib12030045, 2023 (PDF )
Suzuki H, Kitamura K, Goto N, Ishikawa K, Ouchida T, Tanaka T, Kaneko MK, Kato Y.
Int. J. Mol. Sci. , 24(9), 8411; https://doi.org/10.3390/ijms24098411, 2023 (PDF )
Kudo Y, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
antibodies , 12(2), 31, https://doi.org/10.3390/antib12020031, 2023 (PDF )
Isoda Y, Tanaka Y, Suzuki H, Asano T, Kitamura K, Kudo Y, Ejima R, Ozawa K, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(2), 73–76, https://doi.org/10.1089/mab.2022.0035, 2023 (PDF )
Tateyama N, Asano T, Tanaka T, Isoda T, Okada Y, Kobayashi H, Li G, Nanamiya R, Yoshikawa T, Kaneko MK, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(2), 68–72, https://doi.org/10.1089/mab.2022.0034, 2023 (PDF )
Suzuki H, Ozawa K, Tanaka T, Kaneko MK, Kato Y.
Biomedicines , 11(4), 1099; https://doi.org/10.3390/biomedicines11041099, 2023 (PDF )
Li G, Suzuki H, Tanaka T, Asano T, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 41–47, doi: 10.1089/mab.2022.0031, 2023 (PDF )
Kobayashi H, Asano T, Suzuki H, Tanaka T, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 15–21, https://doi.org/10.1089/mab.2022.0032, 2022 (PDF )
Nanamiya R, Ohishi T, Suzuki H, Mizuno T, Yoshikawa T, Asano T, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 27–33, doi:10.1089/mab.2022.0022, 2022 (PDF )
Suzuki H, Asano T, Ohishi T, Yoshikawa T, Suzuki H, Mizuno T, Tanaka T, Kawada M, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 34–40, doi: 10.1089/mab.2022.0023, 2022 (PDF )
Isoda Y, Tanaka T, Suzuki H, Asano T, Yoshikawa T, Kitamura K, Kudo Y, Ejima R, Ozawa K, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 42(1), 22–26, doi: 10.1089/mab.2022.0029, 2022 (PDF )
Suzuki H, Tanaka T, Goto N, Kaneko MK, Kato Y.
Curr. Issues Mol. Biol. , 45(3), 1875-1888; https://doi.org/10.3390/cimb45030121, 2023 (PDF )
Ejima R, Suzuki H, Tanaka T, Asano T, Kaneko MK, Kato Y.
Int. J. Mol. Sci. , 24(4), 4007; https://doi.org/10.3390/ijms24044007, 2023 (PDF )
Kobayashi H, Asano T, Tanaka T, Suzuki H, Kaneko MK, Kato Y.
antibodies , 12(1), 11; https://doi.org/10.3390/antib12010011, 2023 (PDF )
29. Li G, Suzuki H, Ohishi T, Asano T, Tanaka T, Yanaka M, Nakamura T, Yoshikawa T, Kawada M, Kaneko MK, Kato Y.
Int J Mol Med , 51(2), 18, https://doi.org/10.3892/ijmm.2023.5221, 2023 (PDF )
28. Ohishi T, Kaneko MK, Yoshida Y, Takashima A, Kato Y, Kawada M.
Int. J. Mol. Sci. , 24(2), 1702; https://doi.org/10.3390/ijms24021702, 2023 (PDF )
2022
Asano T, Tanaka T, Suzuki H, Li G, Nanamiya R, Tateyama N, Isoda Y, Okada Y, Kobayashi H, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 343–349, doi: 10.1089/mab.2022.0021, 2022 (PDF )
Tanaka T, Suzuki H, Asano T, Li G, Nanamiya R, Tateyama N, Isoda Y, Okada Y, Kobayashi H, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 339–342, doi:10.1089/mab.2022.0020, 2022 (PDF )
Saito M, Suzuki H, Tanaka T, Asano T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 333–338, https://doi.org/10.1089/mab.2021.0069, 2022 (PDF )
Kawabata H, Ohishi T, Suzuki H, Asano T, Kawada M, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 320–327, https://doi.org/10.1089/mab.2021.0049, 2022 (PDF )
Nanamiya R, Suzuki H, Takei J, Li G, Goto N, Harada H, Saito M, Tanaka T, Asano T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 311–319, https://doi.org/10.1089/mab.2021.0058, 2022 (PDF )
Tanaka T, Suzuki H, Isoda Y, Asano T, Nakamura T, Yanaka M, Handa S, Takahashi N, Okuno S, Yoshikawa T, Li G, Nanamiya R, Goto N, Tateyama N, Okada Y, Kobayashi H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(6), 303–310, doi: 10.1089/mab.2022.0027, 2022 (PDF )
Tateyama N, Asano T, Suzuki H, Li G, Yoshikawa T, Tanaka T, Kaneko MK, Kato Y.
antibodies , 11(4), 75; https://doi.org/10.3390/antib11040075, 2022 (PDF )
Asano T, Tanaka T, Suzuki H, Li G, Ohishi T, Kawada M, Yoshikawa T, Kaneko MK, Kato Y.
antibodies , 11(4), 74; https://doi.org/10.3390/antib11040074, 2022 (PDF )
Tateyama N, Suzuki H, Ohishi T, Asano T, Tanaka T, Mizuno T, Yoshikawa T, Kawada M, Kaneko MK, Kato Y.
pharmaceutics ,14(11), 2494; https://doi.org/10.3390/pharmaceutics14112494, 2022 (PDF )
Isoda Y, Tanaka T, Suzuki H, Asano T, Nakamura T, Yanaka M, Handa S, Komatsu Y, Okuno S, Takahashi N, Okada Y, Kobayashi H, Li G, Nanamiya R, Goto N, Tateyama N, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,41(5),275–278, DOI: 10.1089/mab.2022.0019, 2022 (PDF )
Tanaka T, Suzuki H, Li G, Nanamiya R, Isoda Y, Okada Y, Kobayashi H, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,41(5),285–289, DOI: 10.1089/mab.2022.0018, 2022 (PDF )
Saito M, Suzuki H, Asano T, Tanaka T, Yoshikawa T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. ,41(5),279–284, https://doi.org/10.1089/mab.2022.0016, 2022 (PDF )
Ishikawa A, Waseda M, Ishii T, Kaneko MK, Kato Y, Kaneko S.
Genes Cells, 27(9), 549-558, https://doi.org/10.1111/gtc.12972, 2022 (PDF )
Tanaka T, Li G, Saito M, Suzuki H, Asano T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(4), 188–193, https://doi.org/10.1089/mab.2022.0001, 2022 (PDF )
Kudo Y, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(4), 194–201, https://doi.org/10.1089/mab.2022.0007, 2022 (PDF )
Asano T, Suzuki H, Tanaka T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(4), 214–220, https://doi.org/10.1089/mab.2022.0015, 2022 (PDF )
Okada Y, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(4), 221–227, https://doi.org/10.1089/mab.2022.0017, 2022 (PDF )
Suzuki H, Ohishi T, Asano T, Tanaka T, Saito M, Mizuno T, Yoshikawa T, Kawada M, Kaneko MK, Kato Y.
Oncol. Rep., ,48(3), 154, https://doi.org/10.3892/or.2022.8366, 2022 (PDF )
Saito M, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(3), 157–162, https://doi.org/10.1089/mab.2022.0013, 2022 (PDF )
8. Tanaka T, Li G, Asano T, Kaneko MK, Suzuki H, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(3), 150–156, https://doi.org/10.1089/mab.2022.0012, 2022 (PDF )
7. Goto N, Suzuki H, Tanaka T, Asano T, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(3), 163–169, https://doi.org/10.1089/mab.2022.0014, 2022 (PDF )
6. Kitamura K, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , 41(3), 133–141, https://doi.org/10.1089/mab.2022.0010, 2022 (PDF )
5. Li G, Suzuki H, Asano T, Tanaka T, Suzuki H, Kaneko MK, Kato Y.
Antibodies , 11(2), 41; https://doi.org/10.3390/antib11020041, 2022 (PDF ) (Preprint )
4. Goto N, Suzuki H, Tanaka T, Asano T, Kaneko MK, Kato Y.
Int. J. Mol. Sci., 23(10), 5535; https://doi.org/10.3390/ijms23105535, 2022 (PDF ) (Preprint )
2021
Asano T, Suzuki H, Kaneko MK, Kato Y.
Monoclon. Antib. Immunodiagn. Immunother. , DOI: 10.1089/mab.2021.0030, 2021 (ref )2020
Kaneko MK, Ohishi T, Kawada M, Kato Y.
Biochem Biophys Rep., 24, 100826, https://doi.org/10.1016/j.bbrep.2020.100826, 2020 (PDF )Kaneko MK, Ohishi T, Nakamura T, Inoue H, Takei J, Sano M, Asano T, Sayama Y, Hosono H, Suzuki H, Kawada M, Kato Y.* Monoclon. Antib. Immunodiagn. Immunother. , 39(5), 167–174, https://doi.org/10.1089/mab.2020.0019, 2020 (PDF )
動画(YouTube)
総説(pdf)
AMED project